- Magnesium Mass Number 25
- Periodic Table Magnesium Mass Number
- Magnesium Protons Neutrons And Electrons
- Magnesium-24 Mass Number
- What Is Magnesium Mass Number
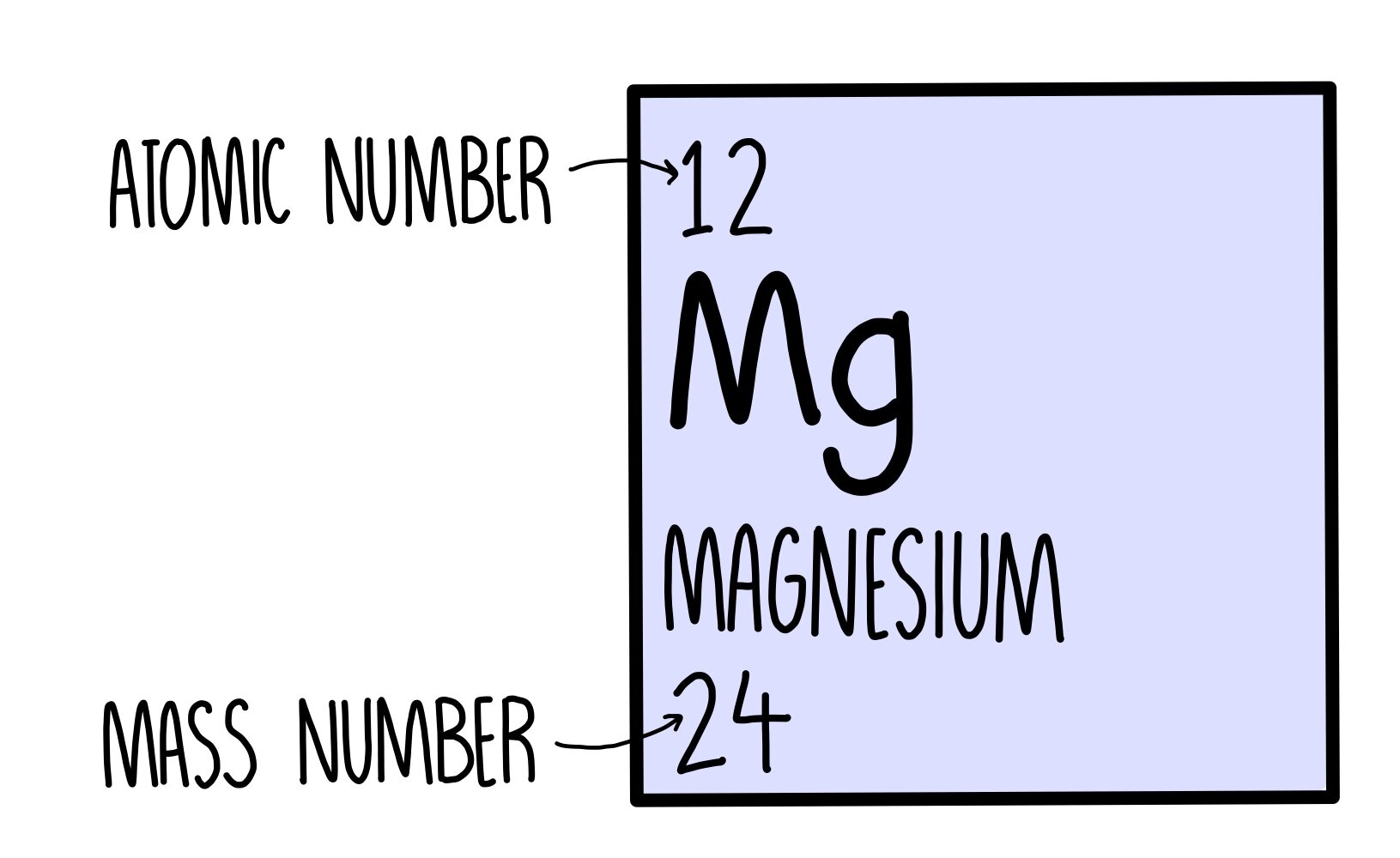
The neutrons are a different matter. Magesium's average atomic mass is 24.305 atomic mass units, but no magnesium atom has exactly this mass.
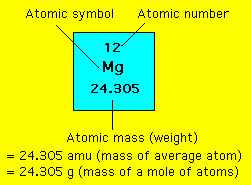
WebMD - Better information. The most stable isotope of magnesium has a mass number of 24 and an atomic number 12. Mass number is the sum of the number of protons and neutrons in the nucleus of an atom while atomic number indicates the number of protons. Normal serum magnesium concentrations range between 0.75 and 0.95 millimoles (mmol)/L 1, 5. Hypomagnesemia is defined as a serum magnesium level less than 0.75 mmol/L 6. Magnesium homeostasis is largely controlled by the kidney, which typically excretes about 120 mg magnesium into the urine each day 2. The mass number is the number of protons plus neutrons. Magnesium is element number 12, so it has 12 protons. Its mass number with 14 neutrons is 12 + 14 which is 26. What is the valency of an atom.
Atomic masses like the one quoted above are found by taking an average of the masses of each isotope, weighted based on how much of each is present in nature.
An isotope is a compound with the same number of protons and electrons, but different number of neutrons.
The three most natural isotopes of Mg are Mg-24, Mg-25, and Mg-26.
Magnesium Mass Number 25
Mg-24 (12 neurtrons) is 78.9%, Mg-25 (13 neutrons) is 10% and Mg-26 (14 neutrons) is 11.01%, of all the Magnesium found in nature. There are also synthetic isotopes, created as byproducts of nuclear decay or intentionally for commercial use, so they aren't included.
So you might account for this isotope problem by saying that about 79% of all Magnesium atoms have 12 neutrons, 12 protons, and 12 electrons.
Periodic Table Magnesium Mass Number
For further research, I suggest you use the source I used to obtain this information (available at your local library):Magnesium Protons Neutrons And Electrons
Jason
Magnesium-24 Mass Number

What Is Magnesium Mass Number
(published on 10/22/2007)
