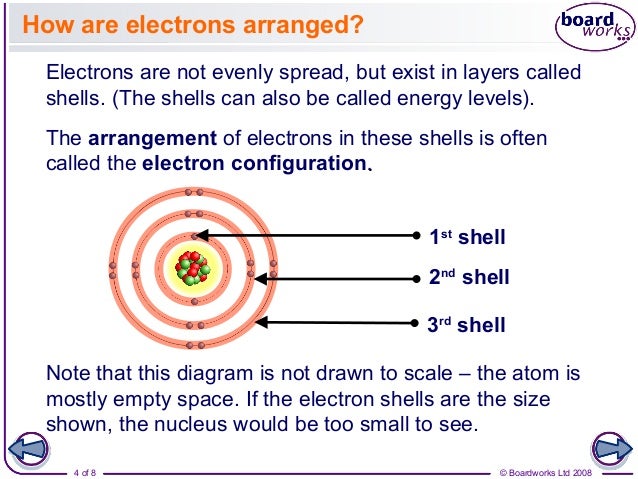
Electron Configuration
- See Full List On Wikihow.com
- Number Of Valence Electrons Chart
- Valence Electrons Are Electrons Located
The electrons in an atom fill up its atomic orbitals according to the Aufbau Principle; 'Aufbau,' in German, means 'building up.' The Aufbau Principle, which incorporates the Pauli Exclusion Principle and Hund's Rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals:
Valence electrons are electrons located A) in the outermost energy level of an atom B) in the nucleus of an atom C) in the innermost energy level of an atom D) throughout the atom E) in the first three shells of an atom Amylose is a form of starch which has as- A) only -1,4-bonds between glucose units B) only -1,4-links bonds glucose units C) both -1,4-and -1,4-bonds between glucose units D. Valence Electrons are the electrons that are located furthest away from the atom itself in the outermost electron shell. They are located on the last energy level also known as the valence level. Valence electrons are electrons present in the outermost orbitals of an atom. These are the electrons that have the least attraction towards the nucleus of an atom. This is because valence electrons are located in a long distance than other electrons of that atom.
- Electrons always fill orbitals of lower energy first. 1s is filled before 2s, and 2s before 2p.
- The Pauli Exclusion Principle states no two electrons within a particular atom can have identical quantum numbers. In function, this principle means that if two electrons occupy the same orbital, they must have opposite spin.
- Hund's Rule states that when an electron joins an atom and has to choose between two or more orbitals of the same energy, the electron will prefer to enter an empty orbital rather than one already occupied. As more electrons are added to the atom, these electrons tend to half-fill orbitals of the same energy before pairing with existing electrons to fill orbitals.
Valency and Valence Electrons
The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. Valence electrons are the highest energy electrons in an atom and are therefore the most reactive. While inner electrons (those not in the valence shell) typically don't participate in chemical bonding and reactions, valence electrons can be gained, lost, or shared to form chemical bonds. For this reason, elements with the same number of valence electrons tend to have similar chemical properties, since they tend to gain, lose, or share valence electrons in the same way. The Periodic Table was designed with this feature in mind. Each element has a number of valence electrons equal to its group number on the Periodic Table. This table illustrates a number of interesting, and complicating, features of electron configuration.
First, as electrons become higher in energy, a shift takes place. Up until now, we have said that as the principle quantum number, increases, so does the energy level of the orbital. And, as we stated above in the Aufbau principle, electrons fill lower energy orbitals before filling higher energy orbitals. However, the diagram above clearly shows that the 4s orbital is filled before the 3d orbital. In other words, once we get to principle quantum number 3, the highest subshells of the lower quantum numbers eclipse in energy the lowest subshells of higher quantum numbers: 3d is of higher energy than 4s.
Second, the above indicates a method of describing an element according to its electron configuration. As you move from left to right across the periodic table, the above diagram shows the order in which orbitals are filled. If we were the actually break down the above diagram into groups rather than the blocks we have, it would show how exactly how many electrons each element has. For example, the element of hydrogen, located in the uppermost left-hand corner of the periodic table, is described as 1s1, with the s describing which orbital contains electrons and the 1 describing how many electrons reside in that orbital. Lithium, which resides on the periodic table just below hydrogen, would be described as 1s22s1. The electron configurations of the first ten elements are shown below (note that the valence electrons are the electron in highest energy shell, not just the electrons in the highest energy subshell).
The Octet Rule
Our discussion of valence electron configurations leads us to one of the cardinal tenets of chemical bonding, the octet rule. The octet rule states that atoms becomeespecially stable when their valence shells gain a full complement of valence electrons. For example, in above, Helium (He) and Neon (Ne) have outer valence shells that are completely filled, so neither has a tendency to gain or lose electrons. Therefore, Helium and Neon, two of the so-called Noble gases, exist in free atomic form and do not usually form chemical bonds with other atoms.
Most elements, however, do not have a full outer shell and are too unstable to exist as free atoms. Instead they seek to fill their outer electron shells by forming chemical bonds with other atoms and thereby attain Noble Gas configuration. An element will tend to take the shortest path to achieving Noble Gas configuration, whether that means gaining or losing one electron. For example, sodium (Na), which has a single electron in its outer 3s orbital, can lose that electron to attain the electron configuration of neon. Chlorine, with seven valence electrons, can gain one electron to attain the configuration of argon. When two different elements have the same electron configuration, they are called isoelectronic.
Diamagnetism and Paramagnetism
The electron configuration of an atom also has consequences on its behavior in relation to magnetic fields. Such behavior is dependent on the number of electrons an atom has that are spin paired. Remember that Hund's Rule and the Pauli Exclusion Principle combine to dictate that an atom's orbitals will all half-fill before beginning to completely fill, and that when they completely fill with two electrons, those two electrons will have opposite spins.
An atom with all of its orbitals filled, and therefore all of its electrons paired with an electron of opposite spin, will be very little affected by magnetic fields. Such atoms are called diagmetic. Conversely, paramagnetic atoms do not have all of their electrons spin-paired and are affected by magnetic fields. There are degrees of paramagnetism, since an atom might have one unpaired electron, or it might have four.
In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level and thus determine the electrical conductivity of the solid. In non-metals, the valence band is the highest range of electronenergies in which electrons are normally present at absolute zero temperature, while the conduction band is the lowest range of vacant electronic states. On a graph of the electronic band structure of a material, the valence band is located below the Fermi level, while the conduction band is located above it.
The distinction between the valence and conduction bands is meaningless in metals, because conduction occurs in one or more partially filled bands that take on the properties of both the valence and conduction bands.
See Full List On Wikihow.com
Band gap[edit]
In semiconductors and insulators the two bands are separated by a band gap, while in semimetals the bands overlap. A band gap is an energy range in a solid where no electron states can exist due to the quantization of energy. Electrical conductivity of non-metals is determined by the susceptibility of electrons to be excited from the valence band to the conduction band.
Electrical conductivity[edit]
Semiconductor band structure
See electrical conduction and semiconductor for a more detailed description of band structure.
In solids, the ability of electrons to act as charge carriers depends on the availability of vacant electronic states. This allows the electrons to increase their energy (i.e., accelerate) when an electric field is applied. Similarly, holes (empty states) in the almost filled valence band also allow for conductivity.
As such, the electrical conductivity of a solid depends on its capability to flow electrons from the valence to the conduction band. Hence, in the case of a semimetal with an overlap region, the electrical conductivity is high. If there is a small band gap (Eg), then the flow of electrons from valence to conduction band is possible only if an external energy (thermal, etc.) is supplied; these groups with small Eg are called semiconductors. If the Eg is sufficiently high, then the flow of electrons from valence to conduction band becomes negligible under normal conditions; these groups are called insulators.
There is some conductivity in semiconductors, however. This is due to thermal excitation—some of the electrons get enough energy to jump the band gap in one go. Once they are in the conduction band, they can conduct electricity, as can the hole they left behind in the valence band. The hole is an empty state that allows electrons in the valence band some degree of freedom.
See also[edit]
- Electrical conduction for more information about conduction in solids, and another description of band structure.
- Semiconductor for a full explanation of the band structure of materials.
References[edit]
- 'Chembio'.
- 'Hyperphysics'.
- Kittel, Charles (2005). Introduction to Solid State Physics. Wiley. ISBN0-471-41526-X.
Number Of Valence Electrons Chart
External links[edit]
Valence Electrons Are Electrons Located
